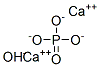Hydroxyapatite: Biological function and applications
General description
Hydroxyapatite is a naturally occurring mineral form of calcium apatite. Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. Up to 50% by volume and 70% by weight of human bone is a modified form of hydroxyapatite, known as bone mineral. Carbonated calcium-deficient hydroxyapatite is the main mineral of which dental enamel and dentin are composed. Hydroxyapatite crystals are also found in the small calcifications, within the pineal gland and other structures, known as corpora arenacea or 'brain sand'.[1] Its appearance is as follows:

Figure 1 Appearance of Hydroxyapatite
Biological function
Hydroxyapatite is present in bone and teeth; bone is made primarily of Hydroxyapatite crystals interspersed in a collagen matrix—65 to 70% of the mass of bone is hydroxyapatite. Similarly, Hydroxyapatite is 70 to 80% of the mass of dentin and enamel in teeth. In enamel, the matrix for Hydroxyapatite is formed by amelogenins and enameling instead of collagen. Hydroxyapatite deposits in tendons around joints result in the medical condition calcific tendinitis.[2]
Applications
Hydroxyapatite can be added to some variations of cornstarch-based baby powder such as Johnson's Aloe & Vitamin E powder. According to the website, the mineral is added as an emollient to "help moisturize and soften skin." Hydroxyapatite is increasingly used to make bone grafting materials as well as dental prosthetics and repairs. Some implants, e.g. hip replacements, dental implants, and bone conduction implants, are coated with hydroxyapatite. As the native dissolution rate of hydroxyapatite in-vivo, around 10 wt% per year, is significantly lower than the growth rate of newly formed bone tissue, in its use as a bone replacement material, ways are sought to enhance its solubility rate and thus promote better bioactivity.[3] Hydroxyapatite is added to special toothpaste as an additive to prevent tooth decay and counteract tooth sensitivity. Microcrystalline hydroxyapatite (MCHA) is marketed as a "bone-building" supplement with superior absorption in comparison to calcium.[4] It is a second-generation calcium supplement derived from bovine bone.[4] In the 1980s, bone meal calcium supplements were found to be contaminated with heavy metals, and although the manufacturers claim their MCHA is free from contaminants, it isn't recommended because its effect on the body has not been well-tested.[4]
In archaeology, hydroxyapatite from human and animal remains can be analyzed to reconstruct ancient diets, migrations, and paleoclimate. The mineral fractions of bone and teeth act as a reservoir of trace elements, including carbon, oxygen, and strontium. Stable isotope analysis of human and faunal hydroxyapatite can be used to indicate whether a diet was predominantly terrestrial or marine in nature (carbon, strontium); the geographical origin and migratory habits of an animal or human (oxygen, strontium) and to reconstruct past temperatures and climate shifts (oxygen). Post-depositional alteration of bone can contribute to the degradation of bone collagen, the protein required for stable isotope analysis.[5]
Hydroxyapatite is a potential adsorbent for the defluorination of drinking water, as it forms fluorapatite in a three-step process. Hydroxyapatite removes F− from the water to replace OH− forming fluorapatite. However, during the defluorination process, the hydroxyapatite, and increase the pH and phosphate ion concentration which makes the defluorinated water unfit for drinking. Recently, a ″calcium amended-hydroxyapatite″ defluorination technique was suggested to overcome the phosphate leaching from hydroxyapatite. This technique can also affect fluorosis reversal by providing calcium-enriched alkaline drinking water to fluorosis-affected areas.
References
[1]Angervall, Lennart; Berger, Sven; R?ckert, Hans (2009). "A Microradiographic and X-Ray Crystallographic Study of Calcium in the Pineal Body and in Intracranial Tumours". Acta Pathologica et Microbiologica Scandinavica. 44 (2): 113–119. doi:10.1111/j.1699-0463.1958.tb01060.x. PMID 13594470.
[2]Carcia, CR; Scibek, JS (March 2013). "Causation and management of calcific tendonitis and periarthritis". Current Opinion in Rheumatology. 25 (2): 204–9. doi:10.1097/bor.0b013e32835d4e85. PMID 23370373. S2CID 36809845.
[3]Zhu, H.; et al. (2018). "Nanostructural insights into the dissolution behavior of Sr-doped hydroxyapatite". Journal of the European Ceramic Society. 38 (16): 5554–5562. arXiv:1910.10610. doi:10.1016/j.jeurceramsoc.2018.07.056. S2CID 105932012.
[4]Straub, D.A. (2007). "Calcium Supplementation in Clinical Practice: A Review of Forms, Doses, and Indications". Nutrition in Clinical Practice. 22 (3): 286–96. doi:10.1177/0115426507022003286. PMID 17507729.
[5]Van Klinken, G. J. (1999). "Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements". Journal of Archaeological Science. 26 (6): 687–695. doi:10.1006/jasc.1998.0385. Archived from the original on 2020-09-13. Retrieved 2017-12-02.
);You may like
Related articles And Qustion
See also
Lastest Price from Hydroxyapatite manufacturers

US $70.00-60.00/kg2024-05-24
- CAS:
- 1306-06-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20Tons

US $25.00-20.00/box2024-05-20
- CAS:
- Min. Order:
- 1box
- Purity:
- 99.8%
- Supply Ability:
- 50000vials